Can’t Find What You Are Looking For? Find Out About Our Bespoke Design Solutions.
Begoodtex Flame Retardant Healthcare Mesh Screen Fabric
SKU: BG-G3-609
- EN 13773 CLASS 1, DIN 4102-B1, NF-P92-503-M
- 280 cm
- 230 gsm
- Flame Retardant
- 100% Polyester
Fabric Introduction
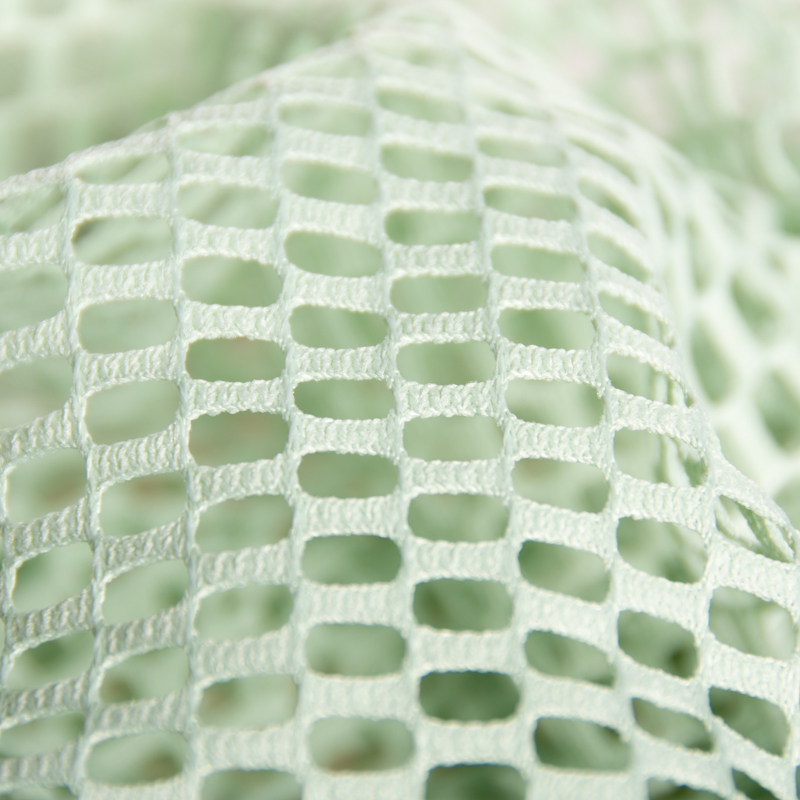
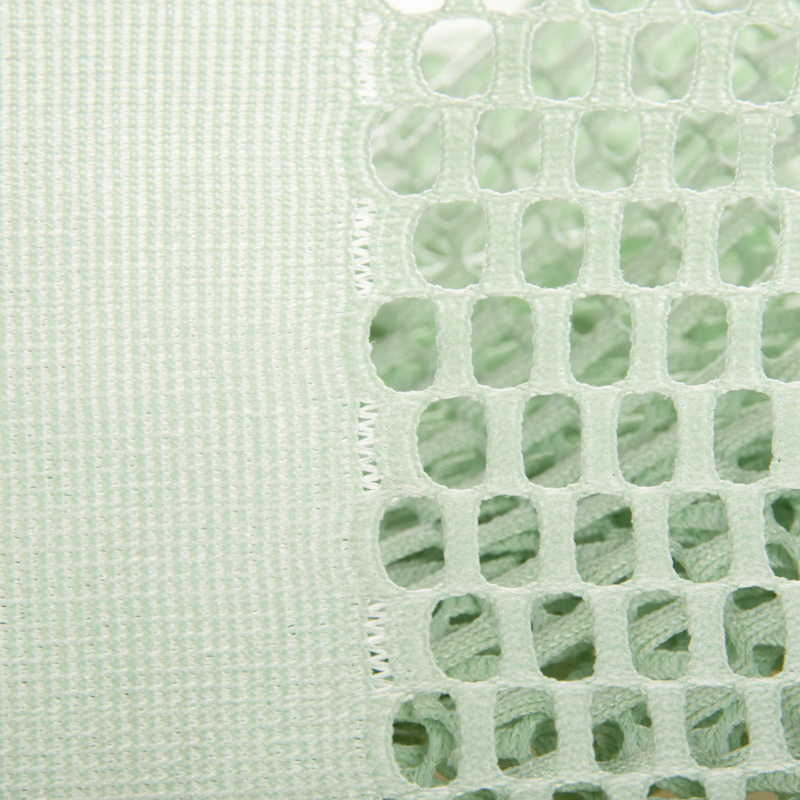
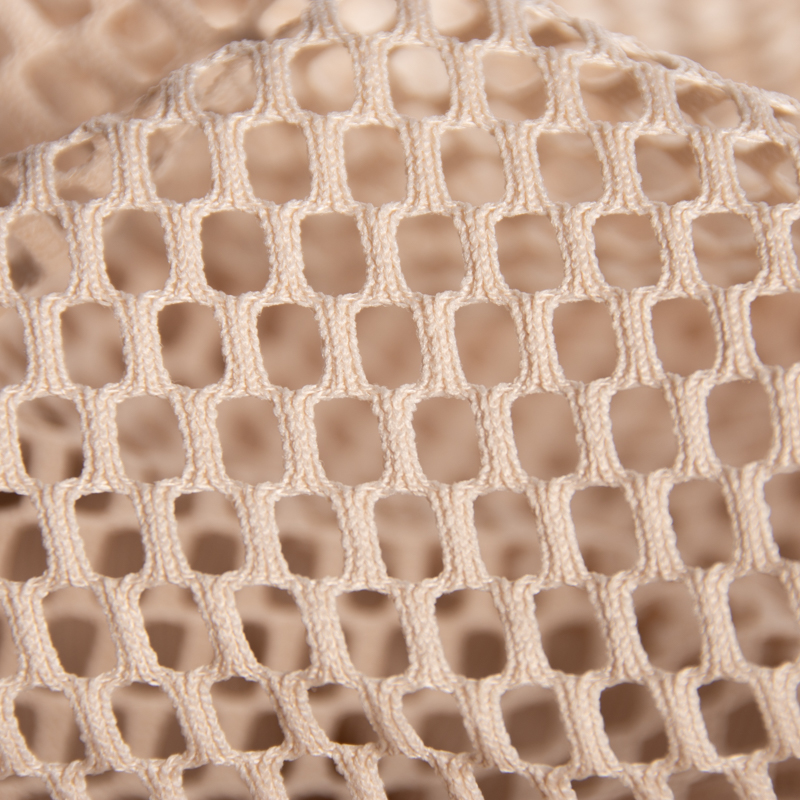
Permanent flame retardant medical curtain fabric is made of begoodtex 100% polyester. This fabric is fruitful in colors. The appearance is the patchwork design. The upper of the fabric is made of mesh knitted fabric, which increase air permeability. The lower part is made of the meshless knitted fabric, it can effectively protect privacy. The fabric is antibacterials, mouldproof, anti-static, anti-UV and easy to clean. Thus can be used in hospitals, clinics, nursing homes and other occasions as the medical curtain.
Fabric Advantages
- Eco-friendly: BEGOODTEX flame retardant medical curtain fabric meets international environmental and recycling standards, which is friendly to human health and the environment.
- Permanent Flame Retardant: Effectively reduces the risk in the event of a fire and does not release toxic gases when exposed to open flames. And it has passed the flame retardant standards of the United States, Germany and many other countries, which can provide you with unparalleled safety assurance.
- Anti-mould and anti-bacterial: Prevents bacteria, stains, unpleasant odors and liquids from spreading between different areas.
- Protection of privacy: Good blackout performance, can effectively saperate the space and protect personal privacy.
Fabric specification:
- Ingredients: 100% flame retardant polyester
- Width: 280cm
- Weight: 230gsm2
- Flame Retardant Standard: NFPA701, DIN4102-B1
- International Standard: Global Recycled Standard
- Function: Flame retardant, antibacterials, mouldproof, anti-static, anti-UV
- Color: Customizable
Fabrics 2 major uses:
This flame retardant medical curtain fabric is especially suitable for the following places:
- Hospitals, clinics, nursing homes, rehabilitation centers: These are the most common uses. In the hospital, the medical curtain can not only divide the wards, operating rooms and other areas, but also can be used in the doctor’s diagnostic room for privacy protection. In addition, during the epidemic of infectious diseases, the use of isolation curtains can effectively avoid the spread of pathogens and protect the health and safety of patients and medical staff.
- Home decoration: Its pattern and color plays a certain effect of decorating the space.
Test Report Summary
Report No: SHIN2007042812SC
Date: July 30, 2020
Manufacturer: SHAOXING BEGOODTEX CO., LTD.
Sample: Inherent flame retardant & anti-bacterial cubicle curtain fabric
Composition: 100% Polyester
Color: Blue
Test 1: NFPA 701-2019 (Flame Propagation of Textiles and Films – Method 1)
Material Details:
- Fabric Weight: 245 g/m²
- Conditioning: Dry at (105±3)℃ for 30 minutes
- No refurbishing/weathering required
Criteria:
- No specimen shall continue burning for more than 2 seconds after falling.
- Average weight loss ≤ 40%.
- No individual specimen’s weight loss shall deviate more than 3 standard deviations from the mean.
Results:
- Conclusion: PASS – The sample met the requirements for NFPA 701-2019 Method 1.
- Average Mass Loss: 10.9%
- Standard Deviation (3x): 5.7%
- Maximum Allowable Loss: 16.6%
Test 2: ASTM E 2149-2013a (Antimicrobial Activity under Dynamic Contact)
Test Organisms:
- Escherichia coli ATCC 25922
- Staphylococcus aureus ATCC 6538
- Klebsiella pneumoniae ATCC 4352
Results:
- Reduction in Bacteria: >99% for all organisms after 24-hour contact.
- Control Data:
E. coli: Initial CFU/mL = 1.9×10⁵; After 24 hours = 1.9×10⁶
S. aureus: Initial CFU/mL = 1.7×10⁵; After 24 hours = 1.1×10⁶
K. pneumoniae: Initial CFU/mL = 1.9×10⁵; After 24 hours = 6.8×10⁵
The submitted sample passed the antimicrobial activity test with significant bacterial reduction.
You must be logged in to post a review.


















There are no reviews yet.